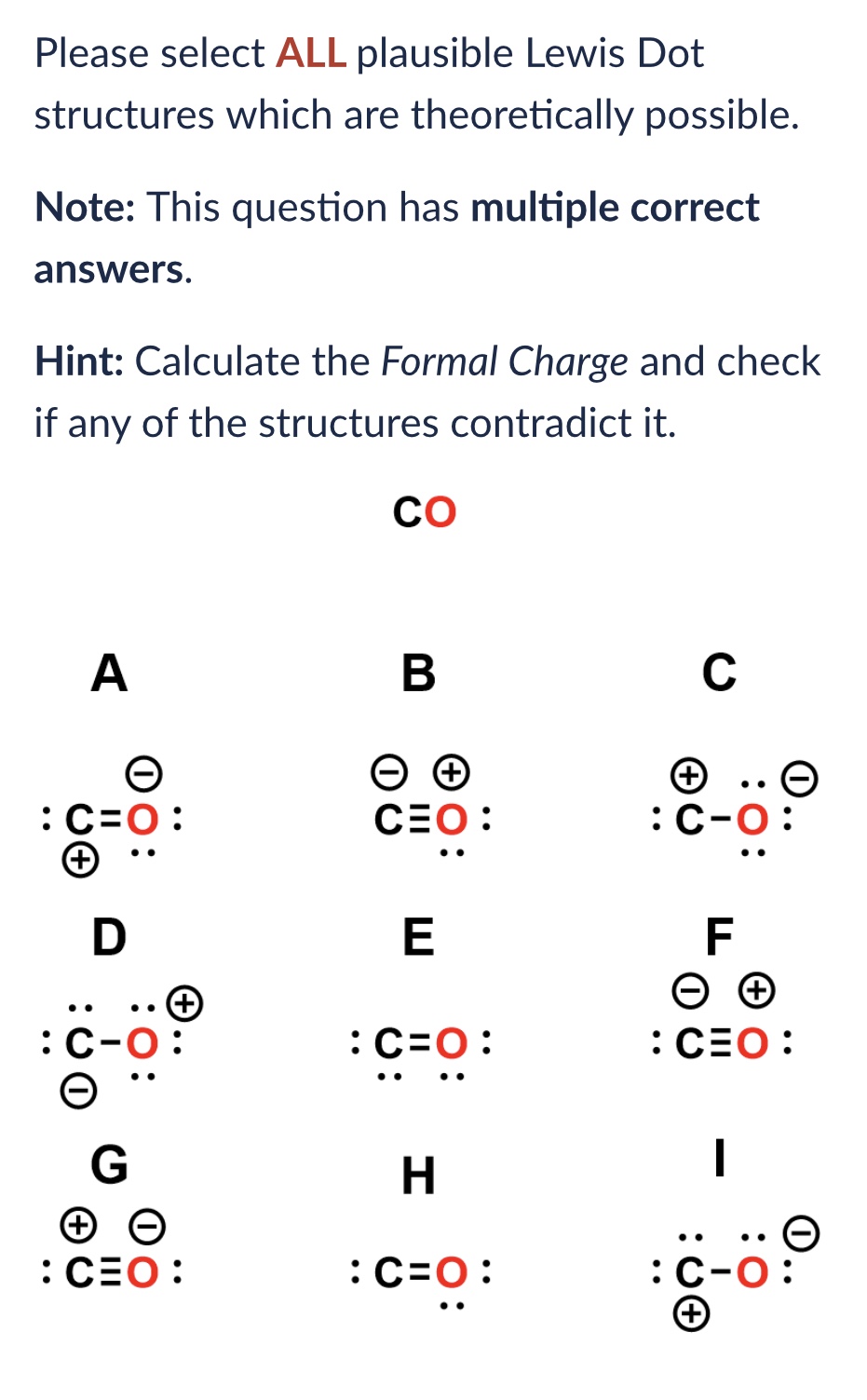
Lewis Dot Structure Notes - When multiple lewis dot structures exist that are differentiated only by the positions of the electrons (the positions of the atoms are. A lewis structure is a way to show how atoms share electrons when they form a molecule. Using your periodic table, check that carbon is in the 4th group. Lewis structures show all of the valence electrons in. You should have 4 total electrons, or dots, drawn in for carbon.
When multiple lewis dot structures exist that are differentiated only by the positions of the electrons (the positions of the atoms are. You should have 4 total electrons, or dots, drawn in for carbon. A lewis structure is a way to show how atoms share electrons when they form a molecule. Using your periodic table, check that carbon is in the 4th group. Lewis structures show all of the valence electrons in.
You should have 4 total electrons, or dots, drawn in for carbon. When multiple lewis dot structures exist that are differentiated only by the positions of the electrons (the positions of the atoms are. A lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in. Using your periodic table, check that carbon is in the 4th group.
Lewis Dot Diagram Worksheet Drawing Lewis Structures Chart
A lewis structure is a way to show how atoms share electrons when they form a molecule. When multiple lewis dot structures exist that are differentiated only by the positions of the electrons (the positions of the atoms are. Using your periodic table, check that carbon is in the 4th group. Lewis structures show all of the valence electrons in..
Structure That Uses An Electron Dot Diagram Lewis Structures
Lewis structures show all of the valence electrons in. A lewis structure is a way to show how atoms share electrons when they form a molecule. Using your periodic table, check that carbon is in the 4th group. You should have 4 total electrons, or dots, drawn in for carbon. When multiple lewis dot structures exist that are differentiated only.
Steps To Follow In Drawing Lewis Structures
A lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in. When multiple lewis dot structures exist that are differentiated only by the positions of the electrons (the positions of the atoms are. Using your periodic table, check that carbon is in the 4th group..
Solved Please select ALL plausible Lewis Dot structures
Lewis structures show all of the valence electrons in. When multiple lewis dot structures exist that are differentiated only by the positions of the electrons (the positions of the atoms are. Using your periodic table, check that carbon is in the 4th group. A lewis structure is a way to show how atoms share electrons when they form a molecule..
Lewis Dot Diagram For C
Lewis structures show all of the valence electrons in. When multiple lewis dot structures exist that are differentiated only by the positions of the electrons (the positions of the atoms are. You should have 4 total electrons, or dots, drawn in for carbon. A lewis structure is a way to show how atoms share electrons when they form a molecule..
How To Draw Lewis Dot Diagrams Simplereality27
When multiple lewis dot structures exist that are differentiated only by the positions of the electrons (the positions of the atoms are. You should have 4 total electrons, or dots, drawn in for carbon. A lewis structure is a way to show how atoms share electrons when they form a molecule. Using your periodic table, check that carbon is in.
Lewis Structures Chart
Lewis structures show all of the valence electrons in. A lewis structure is a way to show how atoms share electrons when they form a molecule. Using your periodic table, check that carbon is in the 4th group. You should have 4 total electrons, or dots, drawn in for carbon. When multiple lewis dot structures exist that are differentiated only.
How to Make a Lewis Structure A Beginner's Guide Best Diy Pro
When multiple lewis dot structures exist that are differentiated only by the positions of the electrons (the positions of the atoms are. You should have 4 total electrons, or dots, drawn in for carbon. Lewis structures show all of the valence electrons in. A lewis structure is a way to show how atoms share electrons when they form a molecule..
Lewis Dot Structure For Molecules Diagrams How To Draw Elect
You should have 4 total electrons, or dots, drawn in for carbon. Using your periodic table, check that carbon is in the 4th group. When multiple lewis dot structures exist that are differentiated only by the positions of the electrons (the positions of the atoms are. Lewis structures show all of the valence electrons in. A lewis structure is a.
Free Printable Lewis Dot Structure Worksheets Worksheets Library
Using your periodic table, check that carbon is in the 4th group. You should have 4 total electrons, or dots, drawn in for carbon. Lewis structures show all of the valence electrons in. A lewis structure is a way to show how atoms share electrons when they form a molecule. When multiple lewis dot structures exist that are differentiated only.
Lewis Structures Show All Of The Valence Electrons In.
You should have 4 total electrons, or dots, drawn in for carbon. A lewis structure is a way to show how atoms share electrons when they form a molecule. Using your periodic table, check that carbon is in the 4th group. When multiple lewis dot structures exist that are differentiated only by the positions of the electrons (the positions of the atoms are.